EPA Researching The Impacts Of Freshwater Salinization Syndrome
By U.S. EPA

Freshwater contains natural salts and minerals. However, dramatic increases in salt concentrations are occurring in freshwaters globally due to human activities including road salt application, water softening, mining and oil production, commercial and industrial processes, weathering of concrete, sea level rise, and fertilizer application. EPA scientists are studying the effects of increasing salinity on environmental health and water quality.
Over the last several decades, EPA scientists and collaborators have measured increases in salt concentration in several rivers around urban areas (Figure 1). Their results show that increasing freshwater salinization is a wide-spread problem in the U.S. and globally.
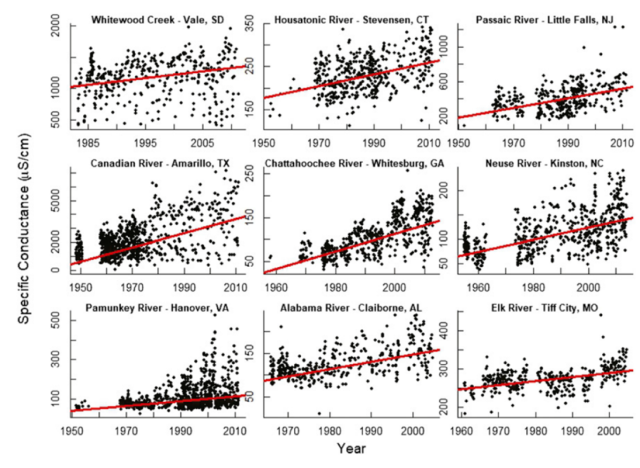
Figure 1. Specific Conductance is an indicator of salt concentration (from Kaushal et al. 2018)
Why Is It A Problem?
Too much salt in freshwater can harm aquatic life. Many different types of salts contribute to freshwater salinization including sodium, chloride, potassium, calcium, and magnesium. Salts can pollute drinking water sources and damage infrastructure. But there's more to the problem than that; increased salt concentrations lead to a phenomenon called freshwater salinization syndrome (FSS). This syndrome is due to direct and indirect effects of salts that cause other pollutants in soil, groundwater, surface water, and water pipes to become more concentrated and mobile (Cooper et al. 2014). One example of these effects is that salts can increase the rate of metals mobilizing from soils and pipes and can cause radioactive materials such as radium in soils to become more concentrated in groundwater and surface water. Excess nutrients in the soil like nitrate-nitrogen can also be mobilized by high salinity, thereby exacerbating nutrient pollution, which contributes to harmful algal blooms and low dissolved oxygen levels in lakes and rivers. Taken together, excess salts can make water undrinkable, increase the cost of treating water, and harm freshwater fish and wildlife.
Excess Salts Create Chemical Cocktails
Excess salts themselves also pose risks to public health, especially for those sensitive to salts or on low salt diets. Salts can corrode metals and exacerbate metal contamination in drinking water, increase nutrient and heavy metal contamination in streams and lakes, and can cause environmental stress to sensitive species. When salts mobilize heavy metals, nutrients, and radionuclides, they can create even more potent “chemical cocktails” which are mixtures of chemicals that may have synergistic toxic effects that may be difficult to treat and remove (Figure 2). Salts and the associated chemical cocktails build up in soils, surface water, and groundwater and are not easily remediated.
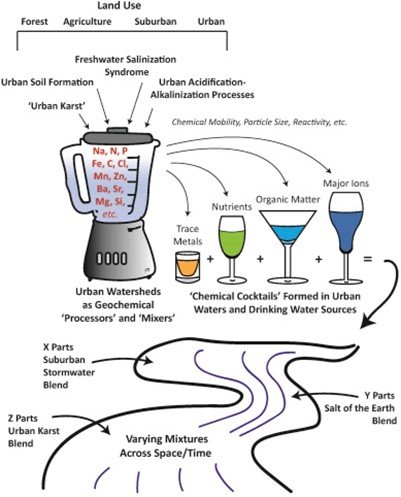
Figure 2. How salts form chemical cocktails (from Kaushal et al. 2020)
Measuring The Effects
EPA scientists and collaborators have developed a five-level scorecard to describe the various causes and potential effects of freshwater salinization syndrome on water quality (Figure 3). This approach is designed to categorize the diversity of chemical contaminants contributing to FSS and to help diagnose the progressive degradation of ecosystems polluted by excess salts. This scoring system does not correspond to any regulatory framework but rather, is an informal gage of environmental condition and therefore, can be applied to any ecosystem type or drinking water source:
- Stage 0: Highest water quality, minimally disturbed
- Stage I: Abnormally elevated concentrations of one or more salt ions across one season
- Stage II: Chronically elevated concentrations of salts ions across multiple seasons
- Stage III: Formation of harmful chemical cocktails exceeding water quality thresholds
- Stage IV: Systems-level failures in infrastructure and ecosystem functions and services (e.g. drinking water and biodiversity)
The above scorecard is intended to be used by watershed and treatment plant managers to assess the stage of FSS of the watershed. Managers could use this as an informal guide to develop local regulations or implement strategies to reduce salts into the waterbody.
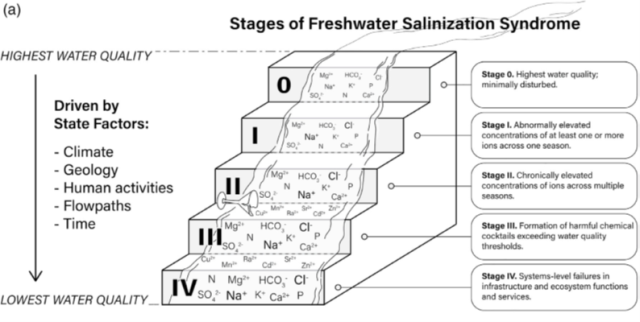
Figure 3. Stages of Freshwater Salinization Syndrome (from Kaushal et al. 2022)
Strategies To Address The Problem
Tackling FSS to reduce the risks to the environment, human health, and the built infrastructure will require multiple approaches based on sound science and effective management techniques. What follows are strategies that can help limit salt inputs and attenuate salts already released to the environment.
- Reducing industrial and agricultural sources. Keeping salts out of waterbodies is the first line of defense in managing negative impacts to ecosystems. Major sources include fertilizers, water softeners, industry, and food processing. Technology and equipment advances, process changes, product choice, and best practices can reduce the volumes of salt used in these applications.
- Managing road salt application and alternatives. Road salt application is a major source of sodium chloride to waterways. Public and private applicators can employ practices to reduce the use of salt such as upgrading salt application equipment and technologies, employing web-based decision-support systems, conducting regular training for vehicle operators on optimal techniques to apply salt most efficiently and effectively, and implementing product alternatives where appropriate.
- Evaluating risk. Preventing and minimizing the impacts FSS depends upon monitoring and assessment by scientists and watershed managers. Risks and vulnerability across the stages of FSS must be evaluated for watersheds, streams, lakes, and groundwater so that prevention and remediation strategies can be identified and prioritized.
- Increase education of the public to spread awareness. Some communities and officials may not be aware that salts can cause water pollution problems or degrade roads and infrastructure. For example, salts can degrade urban infrastructure and bridges, costing taxpayers $800-3,300 for every ton of road salt used (Vitaliano 1992). Damage to drinking water infrastructure from saline water including leaching of lead and heavy metals from pipes has impacted municipal drinking water supplies.
- Federal and State protections. The Clean Water Act (CWA) requires EPA to develop national recommended ambient water quality criteria, or levels of specific pollutants or conditions in a water body that are not expected to cause adverse effects to people (human health criteria) or aquatic organisms (aquatic life criteria). States, territories and tribal governments may use these criteria in their water quality standards regulations or use them as guidance in developing their own criteria. EPA has established numeric aquatic life criteria for some salts, water quality criteria for some salts, such as chloride, to protect aquatic invertebrates and other aquatic organisms. The CWA also authorizes EPA to assist states, territories, and authorized tribes in listing impaired waters and developing Total Maximum Daily Loads (TMDLs) for these waterbodies. A TMDL establishes the maximum amount of a pollutant allowed in a waterbody and serves as a starting point for restoring water quality. Some states are developing TMDLs for chloride as they recognize the problems associated with the salt.
Future EPA Research
EPA researchers are working to understand the magnitude, scale, and scope of Freshwater Salinization Syndrome. EPA’s ongoing and planned research is focused on understanding how stormwater management features may be used to control the release and transport of salts into freshwater. Other EPA research is focused on developing real-time sensor networks to monitor salts in urban streams so that water treatment facilities can better plan for extreme events and pulses of chemical cocktails.
